
Studies of the Earth atmosphere by light scattering and spectroscopy
The Earth atmosphere is of extreme importance for life on Earth. The issues of global warming and ozone depletion form great challenges and scientific understanding is required to seek solutions to these environmental problems. Spectroscopy and light scattering techniques can help to understand the composition of the atmosphere, including the occurence of trace gases, its temperature distribution, the prevailing wind profiles, while its global warming effect can be assessed by investigating photodynamics and photoabsorption processes. Below some of the activities in our group to investigate the Earth atmosphere are highlighted.
1. Light scattering and Rayleigh-Brilouin profiles for measuring the global wind profile
Light scattering of molecules in gases is generally well understood and was described by Rayleigh in his classic paper from 1899. Rayleigh’ s theory, yielding a cross section for light scattering scaling with wavelength (to the power -4), was based on the basic formalism of electromagnetism. The scattering of an incident light beam of of a certain frequency that is propagating through a medium (in our case, the atmosphere) will show, in the most general circumstances, a spectrum that has the form shown in the Figure.
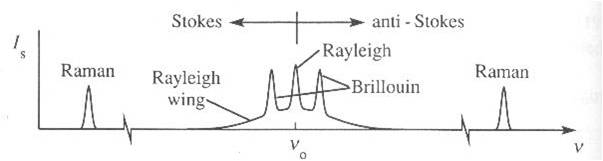
Spectrum of the Rayleigh-scattered radiation
While Rayleigh did not address the specific features in the scatterd light, in the spectrum there is an elastic center-peak also knwon as the Cabannes peak, there are the Raman side peaks associated with rotational and vibrational excitation of the molecule, and there are the Brillouin side peaks associated with acoustic modes in the gas. Even the central Cabannes peak has a certain width, related to the Doppler effect.



Lord Rayleigh Leon Brillouin Raman
We have built a Rayleigh-Brillouin spectrometer in our laboratory to perform measurements on the scattering
profile of gases. For its layout see the figure:
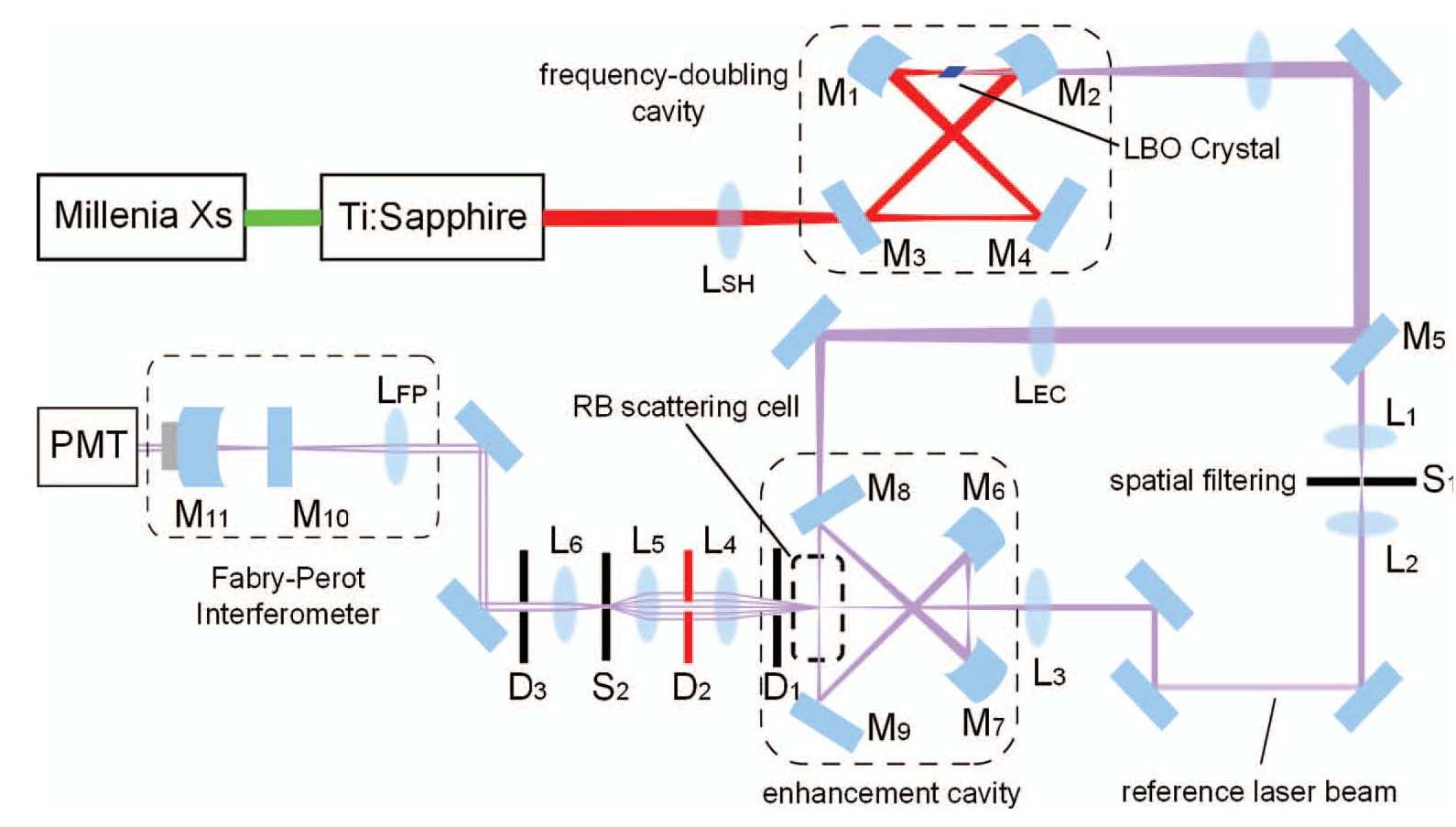
Setup for measuring Rayleigh-Brillouin scattering profiles
The profile of the scattered light, induce by a narrowband laser (1 MHz bandwidth)
is scanned with a Fabry-Perot analyzer. For details see the paper in the
Review of Scientific Instruments.
Goal of the experiments is to test a model developed to describe the full Rayleigh-Brillouin profile
(The TENTI-S6 model) in particular for RB-scattering in air at various pressures and temperatures.
In the figure RB-scattering profiles for dry air at 3 bar and different temperatures are displayed;
also shown is the prediction from the TENTI-model, and below devations between experiment and model are given.
Note that in the model one macroscopic transport coefficient for the gas, the so-called bulk viscosity,
is adapted.
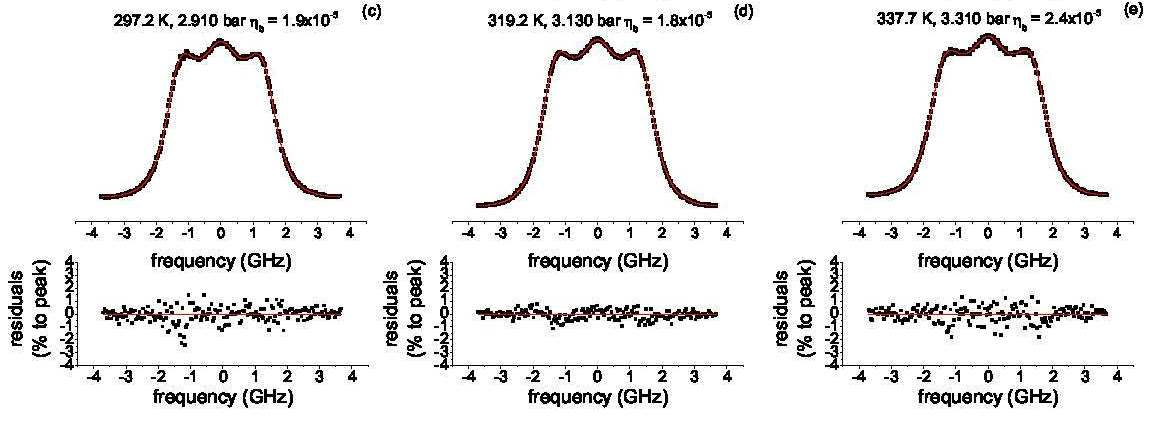
Measured RB-profiles in air under various temperature conditions
These accurate determinations on the scattering profiles are of relevance for modeling the scattered light
in the Earth atmosphere as will be done by the
ADM-Aeolus active remote sensing satellite to be launched soon by ESA. In this mission the wind profile at various layers over the entire globe will be measured from a measurement of the scattered light (taking into account the Doppler shift produced by moving molecules in the atmosphere); also the LIDAR principle is used to probe the various layers ion the atmosphere.
A laser will fly aboard the satellite emitting the light that will be back-scattered and probed by a
detector aboard the satellite.
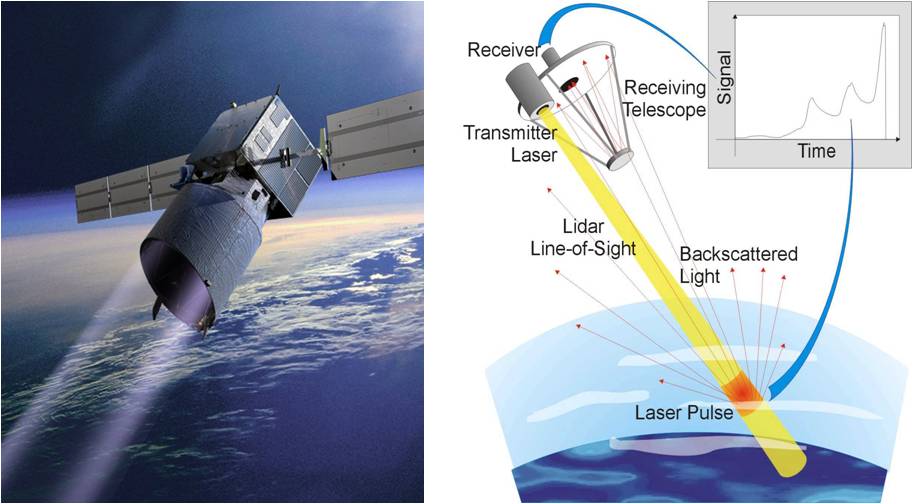
Pictorial view of the ADM-Aeolus satellite and the operating principle (on right)
The laboratory investigations are sponsored by the
European Space Agency in connection with their environmental science program.
See for an extensive report on ESA project ESA - AO/1-5467/07/NL/HE :
A Spontaneous Rayleigh-Brillouin scattering experiment for the characterization of atmospheric
Lidar backscatter.
2. Measurements of absolute cross sections for Rayleigh scattering
The absolute cross section for Rayleigh scattering derives from Rayleigh's treatment based on electromagnetism.
He derived an equation representing the cross section per molecule:
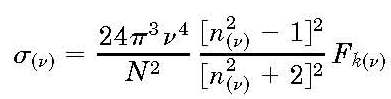
The King factor (Fk) was introduced later and represents a small deviation from Rayleighs theory
associated with depolarized scattering giving rise to a slight enhancement of the cross section,
amounting by a few %.
For many decades the Rayleigh cross section was determined through a measurement of the index of
refraction, mostly via interferometric techniques. In our group, using the technique of
cavity ring-down (CRD) spectroscopy the cross section was determined, for the first time, in a direct manner.
In CRD the light leaking out of a cavity is monitored; the decay rate of the optical cavity in a CRD experiment
is given by:
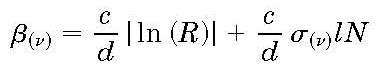
If a cavity is entirely filled with gas the absorption path l is equal
to the length of the cavity d. While monitoring the decay rate of the decay the cell is then continuously pressurized
with gas (increase of density N) the cross section can be directly measured by analyzing the slope of the decay rate vs. density.
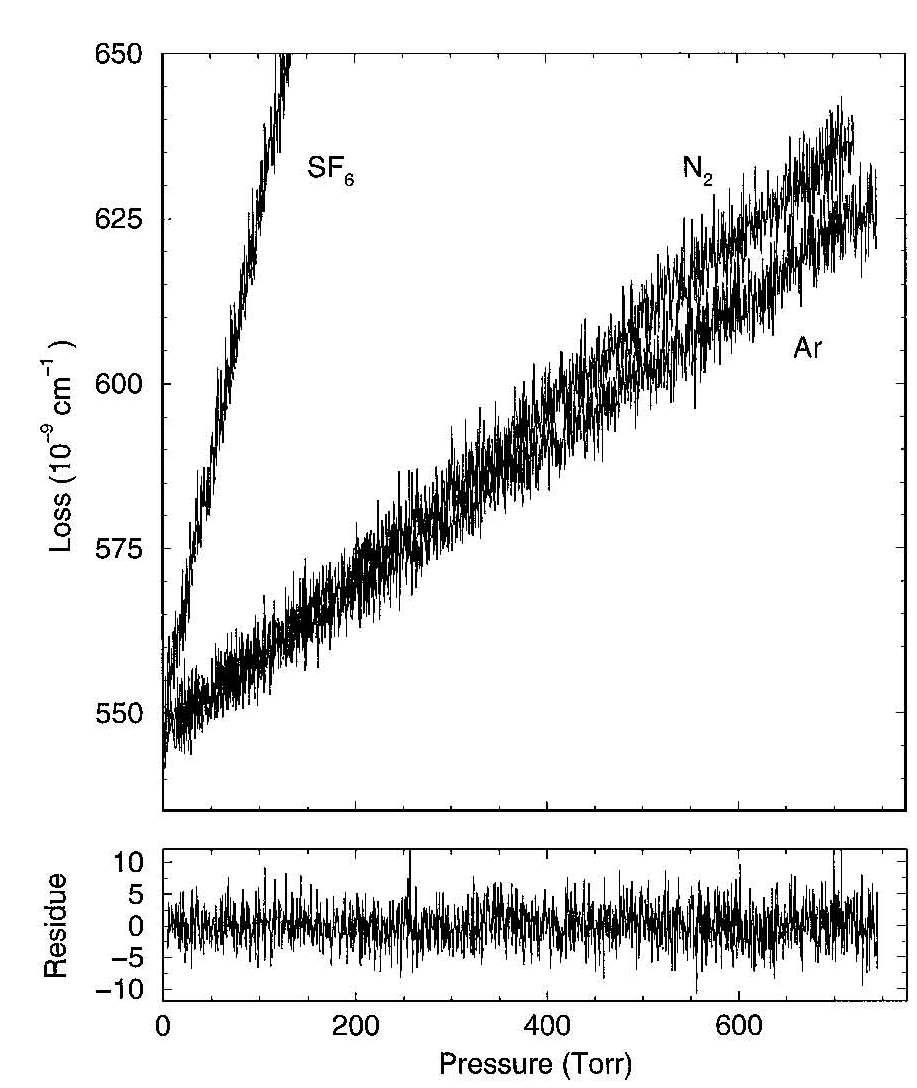
The slope coefficient equals c times the cross section sigma. Below such curves are shown for CRD measurements
for SF6, N2 and Argon gases. the zero density point just represent a value associated with the mirror reflectivity
of the CRD resonator.
This method is the only one so far capable of determining absolute cross sections for Rayleigh scattering in a
direct manner.
For further details see the papers:
H. Naus, W. Ubachs
Experimental Verification of Rayleigh-Scattering Cross sections
Opt. Lett. 25 (2000) 347-349
M. Sneep, W. Ubachs
Direct measurement of the Rayleigh scattering cross section in various gases
J. Quant. Spectr. Rad. Transfer 92 (2005) 293-310
3. Collision-induced absorptions in the Earth atmosphere
4. Photodissociation of atmospheric molecules
Papers on the photo-dissociation spectrum of molecular nitrogen
A.N. Heays, G. Dickenson, E.J. Salumbides, N. de Oliveira, D. Joyeux, L. Nahon,
B.R. Lewis, W. Ubachs,
High-resolution Fourier-transform XUV photoabsorption spectroscopy study of 14N15N
J. Chem. Phys. 135, 244301 (2011)
B.R. Lewis, K.G.H. Baldwin, A.N. Heays, S.T. Gibson, J.P. Sprengers, W. Ubachs,
M. Fujitake,
Structure and predissociation of the 3psu D3Su+
state of N2;
First extreme ultraviolet and new near-infrared observations with coupled-channels analysis
J. Chem. Phys. 129, 204303 (2008)
This paper also appeared in the
Virtual Journal of Ultrafast Science
M.O. Vieitez, T.I. Ivanov, C.A. de Lange, W. Ubachs, A.N. Heays, B.R. Lewis, G. Stark,
Interactions of the 3ppu c1Pu(v=2) Rydberg-complex member in isotopic N2
J. Chem. Phys. 128 134313 (2008)
M.O. Vieitez, T.I. Ivanov, W. Ubachs, B.R. Lewis, C.A. de Lange,
On the complexity of the absorption spectrum of molecular nitrogen
J. Molecular Liquids 141, 110-117 (2008)
M.O. Vieitez, T.I. Ivanov, J.P. Sprengers, C.A. de Lange, W. Ubachs,
B.R. Lewis, G. Stark
Quantum-interference effects in the
o1Pu(v=1) - b1Pu(v=9)
Rydberg-valence complex of molecular nitrogen
Mol. Phys. 105 (2007) 1543-1557
B.R. Lewis, S.T. Gibson, J.P. Sprengers,
W. Ubachs,
A. Johansson, C.-G. Wahlstrom,
Lifetime and predissociation yield of 14N2
b1Pu(v=1) revisited: effects of rotation
J. Chem. Phys. 123 (2005) 236101
J.P. Sprengers,
E. Reinhold,
W. Ubachs,
K.G.H. Baldwin,
B.R. Lewis,
Optical observation of the 3ssg F3Pu
Rydberg state of N2
J. Chem. Phys. 123 (2005) 144315.
J.P. Sprengers,
W. Ubachs,
K.G.H. Baldwin
Isotopic variation of experimental lifetimes for the lowest 1Pu states in N2
J. Chem. Phys. 122 (2005) 144301
J.P. Sprengers, A. Johansson, A. L'Huillier, C.-G. Wahlstrom,
B.R. Lewis, W. Ubachs
Pump-probe lifetime measurements on singlet ungerade states in molecular nitrogen
Chem. Phys. Lett. 389 (2004) 348-351
J.P. Sprengers, W. Ubachs, A. Johansson, A. L'Huillier, C.-G. Wahlstrom,
R. Lang, B.R. Lewis, S.T. Gibson
Lifetime and
predissociation yield of 14N2 b1Piu(v=1)
J. Chem. Phys. 120 (2004) 8973-8978
J.P. Sprengers, W. Ubachs, K.G.H. Baldwin, B.R. Lewis, W.-U.L. Tchang-Brillet
Extreme ultraviolet laser excitation of isotopic molecular nitrogen:
the dipole allowed spectrum of 15N2 and
14N15N
J. Chem. Phys. 119 (2003) 3160-3173
A. de Lange, R. Lang, W.J. van der Zande, W. Ubachs
Highly excited states of gerade symmetry in molecular nitrogen
J. Chem. Phys. 116 (2002) 7893-7901
W. Ubachs, R. Lang, I. Velchev, W.-U L. Tchang-Brillet, A. Johansson,
Z.S. Li, V. Lokhnygin, C.-G. Wahlstrom
Lifetime measurements on the
c4' 1Su+, v=0,1 and 2
states of molecular nitrogen
Chem. Phys. 270 (2001) 215-225
W. Ubachs, I. Velchev, A. de Lange
Predissociation in b1Pu, v (v=1,4,5,6) levels of N2
J. Chem. Phys. 112 (2000) 5611-5616
A. de Lange, W. Ubachs
Observation of the y1Pg-c4'
1Su+
and k1Pg-c4'
1Su+ systems of N2
Chem. Phys. Lett. 310 (1999) 471
W. Ubachs
Predissociative decay of the
c'41Su+,
v=0 state of molecular nitrogen
Chem. Phys. Lett. 268 (1997) 201
W. Ubachs, K.S.E. Eikema, W. Hogervorst
Narrowband extreme ultraviolet radiation tunable in the range 90.5-95 nm:
application to spectroscopy of N2
Appl. Phys. B57 (1993) 411-416
P.F. Levelt, W. Ubachs
XUV-laser spectroscopy on the c4' 1Su+,
v=0 and c3 1Pu, v=0 Rydberg states of N2
Chem. Phys. 163 (1992) 263
W. Ubachs, L.M. Tashiro, and R.N. Zare
Study of the N2 b1Pu state
via 1+1 multiphoton ionization
Chem. Phys. 130 (1989) 1
Papers on the photo-dissociation spectrum of molecular oxygen
S. Hannemann, G.R. Wu, E.-J. van Duijn, W. Ubachs, Ph. Cosby,
Pressure broadening and fine-structure dependent predissociation
in oxygen B3Su-, v=0
J. Chem. Phys. 123, 174318 (2005)
S. Hannemann, E.-J. van Duijn, W. Ubachs,
Deep-UV high resolution cavity ring-down
spectroscopy of the Schumann-Runge bands in
16O2 and 18O2 at wavelengths 197 - 203 nm
J. Mol. Spectr. 232 (2005) 33-38
Papers on the photo-dissociation spectrum of carbon dioxide
D. Ityaksov, H. Linnartz, W. Ubachs,
Deep-UV absorption and Rayleigh scattering of carbon dioxide
Chem. Phys. Lett. 462, 31-34 (2008)
5. Molecular photoabsorption of atmospheric molecules and satellite data retrieval
We are in the process of investigating absorption by the molecular constituents of the Earth's atmosphere, one the one hand the major species, on the other hand trace gases. Laser-based cavity ring-down absorption is applied to determine the spectra of the oxygen atmospheric bands, even including the spectra of the isotopes. An example of a very weak absorption band of O2 is given in the figure (see also
Paper).
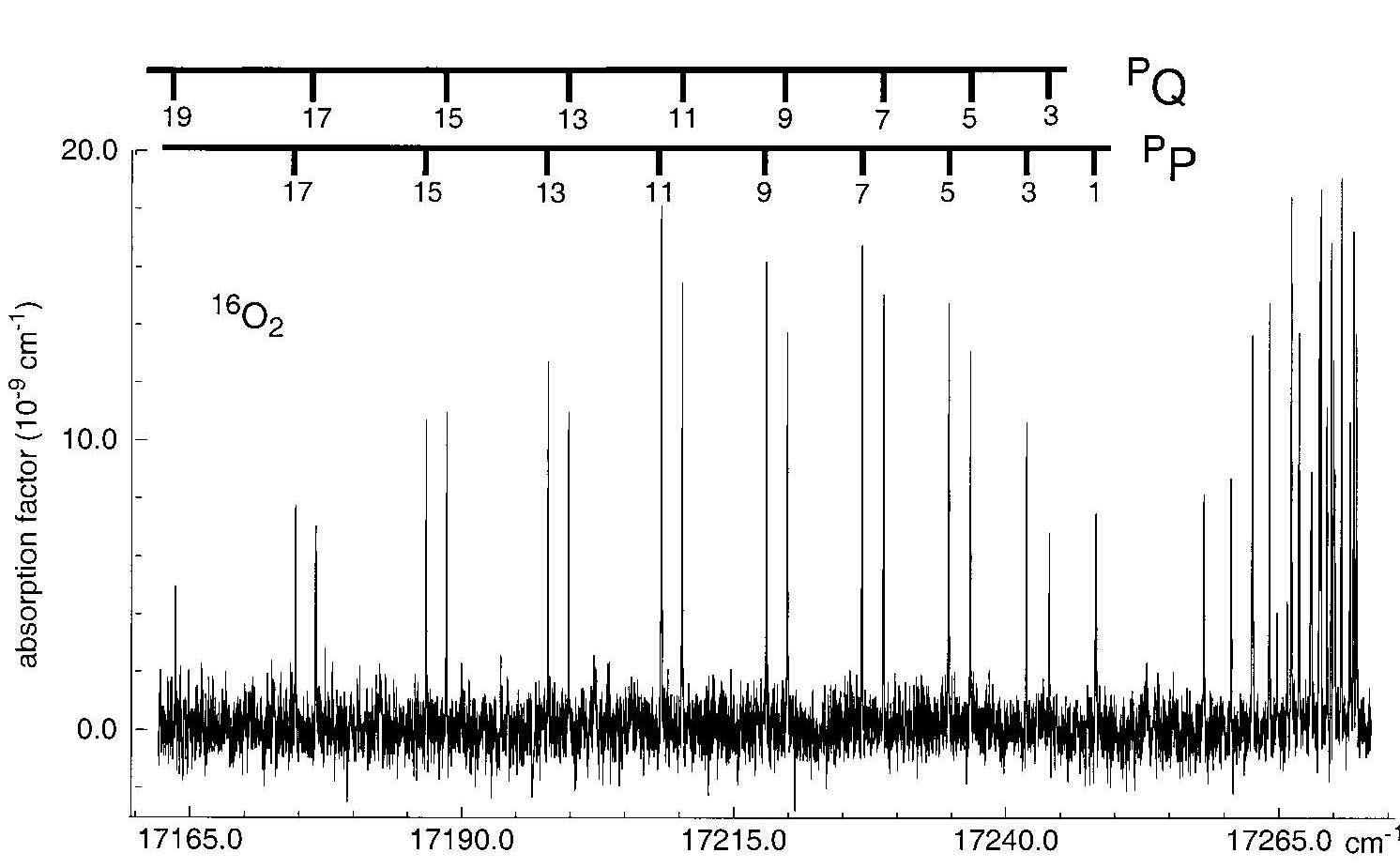
The absorption spectrum of molecular oxygen in the yellow range
Papers on the absorption spectrum of molecular oxygen
H. Naus, A. de Lange, W. Ubachs
b1Sg+ -
X3Sg- (0,0)
band of oxygen isotopomers in relation to tests
of the symmetrization postulate in 16O2
Phys. Rev. A56 (1997) 4755
H. Naus, S.J. van der Wiel, W. Ubachs
Cavity-ring-down spectroscopy on the
b1Sg+ -
X3Sg- (1,0)
band of oxygen isotopomers
J. Mol. Spectr. 192 (1998) 162-168
H. Naus, K. Navaian, W. Ubachs
The gamma-band of 16O2, 16O17O,
17O2 and
18O2
Spectrochimica Acta 55, (1999) 1255
H. Naus, W. Ubachs
The b1Sg+ - X3Sg- (3,0) band of
16O2 and 18O2
J. Mol. Spectr. 193 (1999) 442-445
Water vapour is an extremely important minor constituent in the Earth atmosphere. It contributes for a large fraction to the uptake of infrared radiation making up for a large fraction of the absorption part of the Earth radiation budget. Absorptions in the visible (vibrational overtone bands) are much weaker, but nevertheless contribute. In addition the absorption bands in the visible are of importance, because those can be used for retreival of satellite information. As an example we show the water vapour spectrum in the yellow range.
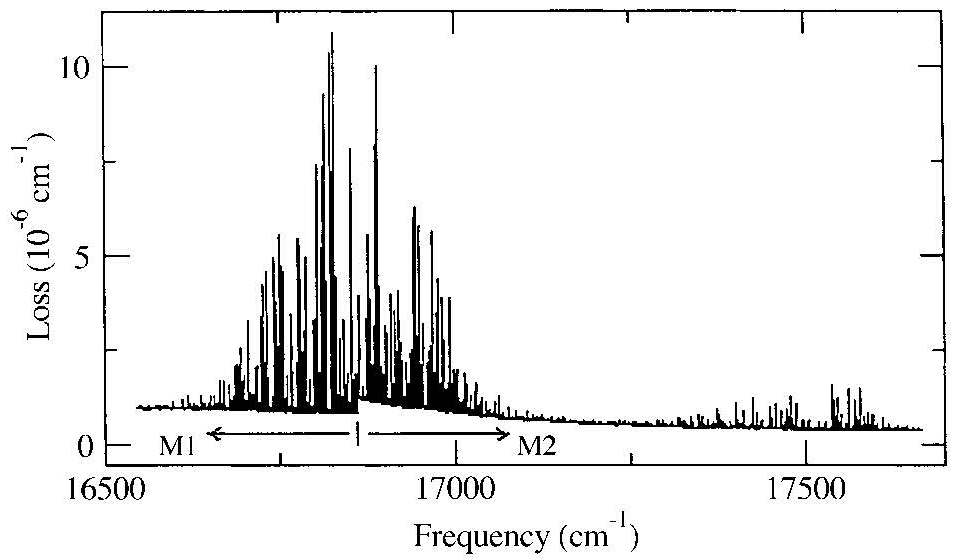
The water vapour absorption spectrum in the yellow wavelength range
These laboratory data on the water vapor absorption spectrum were used in novel retrieval algorithms to determine the column density of water vapor in the Earth atmosphere.
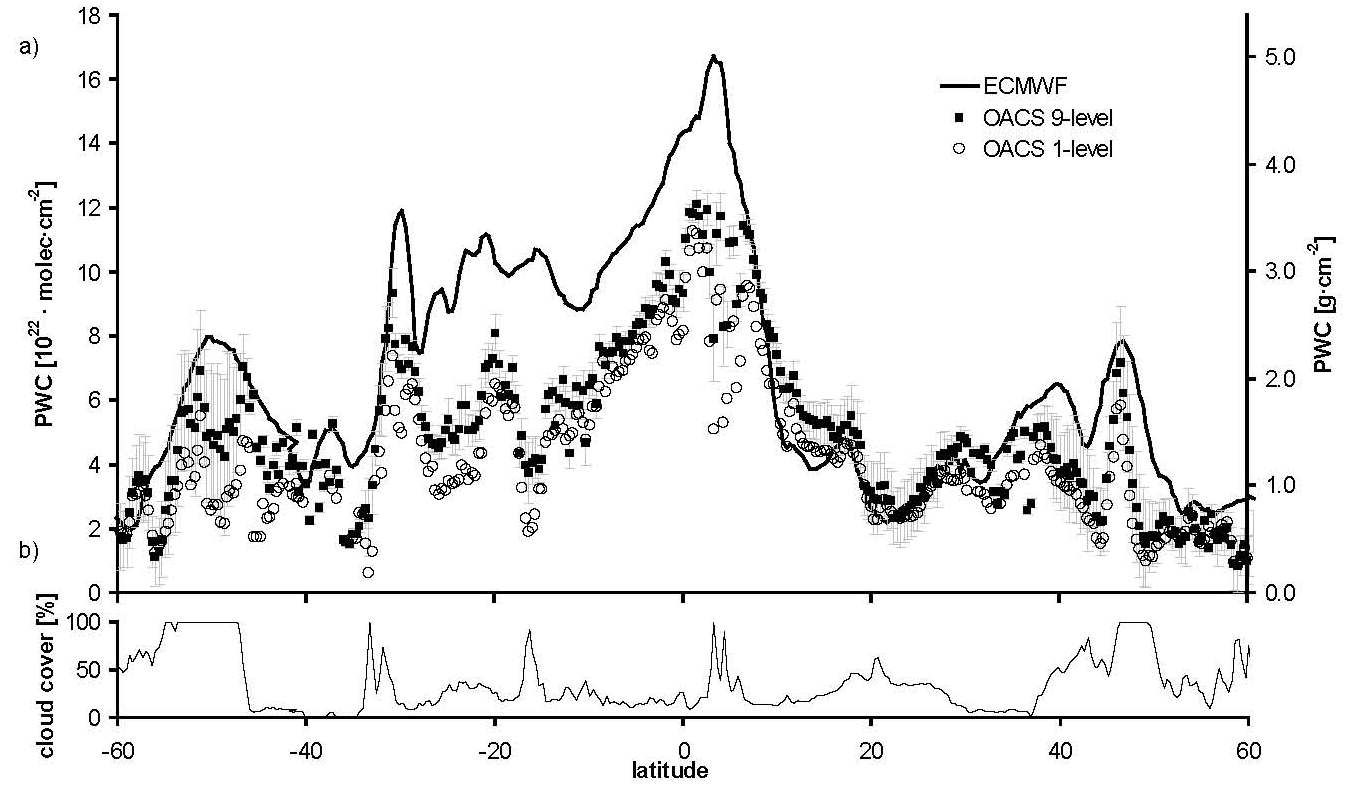
Comparison of GOME-retrieved precipitable water columns with ECMWF expected values
For this study data were used from the
GOME satellite by ESA with a spectrometer aboard looking into nadir.
Papers on the absorption spectrum of water vapor and the O18 and O17 isotopes:
H. Naus, W. Ubachs, P.F. Levelt, O.L. Polyansky, N.F. Zobov, J. Tennyson
Cavity-Ring-Down spectroscopy on water vapour in the range 555-604 nm
J. Mol. Spectr. 205 (2001) 117-121
M. Tanaka, M. Sneep, W. Ubachs, J. Tennyson,
Cavity-Ring-Down Spectroscopy of H218O in the 16570 -- 17120 cm-1 frequency range
J. Mol. Spectr. 226 (2004) 1-6
O. Naumenko, M. Sneep, M. Tanaka, S.V. Shirin, W. Ubachs, J. Tennyson,
Cavity ring-down spectroscopy of H217O in the range 16570 -17125 cm-1
Supplementary Material
J. Mol. Spectr. 237, 63-69 (2006)
Papers on the water vapor data retrieval from GOME satellite data:
A.N. Maurellis, R. Lang, W. van der Zande, I. Aben, W. Ubachs
Precipitable water column retrieval from GOME data
Geoph. Res. Lett. 27 (2000) 903-906
R. Lang, A.N. Maurellis, W.J. van der Zande, I. Aben, J. Landgraf, W. Ubachs
Forward modeling and retrieval of water vapor from GOME:
Treatment of narrow band absorption spectra
J. Geoph. Res. 107 D (2002) doi:10.1029/2001JD001453
Studies of the Earth atmosphere by light scattering and spectroscopy
The Earth atmosphere is of extreme importance for life on Earth. The issues of global warming and ozone depletion form great challenges and scientific understanding is required to seek solutions to these environmental problems. Spectroscopy and light scattering techniques can help to understand the composition of the atmosphere, including the occurence of trace gases, its temperature distribution, the prevailing wind profiles, while its global warming effect can be assessed by investigating photodynamics and photoabsorption processes. Below some of the activities in our group to investigate the Earth atmosphere are highlighted.
1. Light scattering and Rayleigh-Brilouin profiles for measuring the global wind profile
Light scattering of molecules in gases is generally well understood and was described by Rayleigh in his classic paper from 1899. Rayleigh’ s theory, yielding a cross section for light scattering scaling with wavelength (to the power -4), was based on the basic formalism of electromagnetism. The scattering of an incident light beam of of a certain frequency that is propagating through a medium (in our case, the atmosphere) will show, in the most general circumstances, a spectrum that has the form shown in the Figure.
Spectrum of the Rayleigh-scattered radiation
While Rayleigh did not address the specific features in the scatterd light, in the spectrum there is an elastic center-peak also knwon as the Cabannes peak, there are the Raman side peaks associated with rotational and vibrational excitation of the molecule, and there are the Brillouin side peaks associated with acoustic modes in the gas. Even the central Cabannes peak has a certain width, related to the Doppler effect.



Lord Rayleigh Leon Brillouin Raman
We have built a Rayleigh-Brillouin spectrometer in our laboratory to perform measurements on the scattering profile of gases. For its layout see the figure:

Setup for measuring Rayleigh-Brillouin scattering profiles
The profile of the scattered light, induce by a narrowband laser (1 MHz bandwidth) is scanned with a Fabry-Perot analyzer. For details see the paper in the Review of Scientific Instruments.
Goal of the experiments is to test a model developed to describe the full Rayleigh-Brillouin profile (The TENTI-S6 model) in particular for RB-scattering in air at various pressures and temperatures. In the figure RB-scattering profiles for dry air at 3 bar and different temperatures are displayed; also shown is the prediction from the TENTI-model, and below devations between experiment and model are given. Note that in the model one macroscopic transport coefficient for the gas, the so-called bulk viscosity, is adapted.

Measured RB-profiles in air under various temperature conditions
These accurate determinations on the scattering profiles are of relevance for modeling the scattered light in the Earth atmosphere as will be done by the ADM-Aeolus active remote sensing satellite to be launched soon by ESA. In this mission the wind profile at various layers over the entire globe will be measured from a measurement of the scattered light (taking into account the Doppler shift produced by moving molecules in the atmosphere); also the LIDAR principle is used to probe the various layers ion the atmosphere. A laser will fly aboard the satellite emitting the light that will be back-scattered and probed by a detector aboard the satellite.

Pictorial view of the ADM-Aeolus satellite and the operating principle (on right)
The laboratory investigations are sponsored by the European Space Agency in connection with their environmental science program. See for an extensive report on ESA project ESA - AO/1-5467/07/NL/HE :
A Spontaneous Rayleigh-Brillouin scattering experiment for the characterization of atmospheric Lidar backscatter.
2. Measurements of absolute cross sections for Rayleigh scattering
The absolute cross section for Rayleigh scattering derives from Rayleigh's treatment based on electromagnetism. He derived an equation representing the cross section per molecule: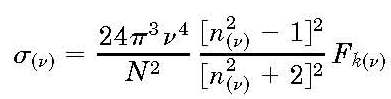
The King factor (Fk) was introduced later and represents a small deviation from Rayleighs theory associated with depolarized scattering giving rise to a slight enhancement of the cross section, amounting by a few %. For many decades the Rayleigh cross section was determined through a measurement of the index of refraction, mostly via interferometric techniques. In our group, using the technique of cavity ring-down (CRD) spectroscopy the cross section was determined, for the first time, in a direct manner. In CRD the light leaking out of a cavity is monitored; the decay rate of the optical cavity in a CRD experiment is given by:
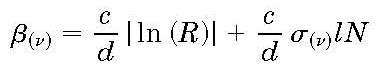
If a cavity is entirely filled with gas the absorption path l is equal to the length of the cavity d. While monitoring the decay rate of the decay the cell is then continuously pressurized with gas (increase of density N) the cross section can be directly measured by analyzing the slope of the decay rate vs. density. The slope coefficient equals c times the cross section sigma. Below such curves are shown for CRD measurements for SF6, N2 and Argon gases. the zero density point just represent a value associated with the mirror reflectivity of the CRD resonator.

This method is the only one so far capable of determining absolute cross sections for Rayleigh scattering in a direct manner. For further details see the papers:
H. Naus, W. Ubachs
Experimental Verification of Rayleigh-Scattering Cross sections
Opt. Lett. 25 (2000) 347-349
M. Sneep, W. Ubachs
Direct measurement of the Rayleigh scattering cross section in various gases
J. Quant. Spectr. Rad. Transfer 92 (2005) 293-310
3. Collision-induced absorptions in the Earth atmosphere
4. Photodissociation of atmospheric molecules

Papers on the photo-dissociation spectrum of molecular nitrogen
A.N. Heays, G. Dickenson, E.J. Salumbides, N. de Oliveira, D. Joyeux, L. Nahon,
B.R. Lewis, W. Ubachs,
High-resolution Fourier-transform XUV photoabsorption spectroscopy study of 14N15N
J. Chem. Phys. 135, 244301 (2011)
B.R. Lewis, K.G.H. Baldwin, A.N. Heays, S.T. Gibson, J.P. Sprengers, W. Ubachs,
M. Fujitake,
Structure and predissociation of the 3psu D3Su+
state of N2;
First extreme ultraviolet and new near-infrared observations with coupled-channels analysis
J. Chem. Phys. 129, 204303 (2008)
This paper also appeared in the
Virtual Journal of Ultrafast Science
M.O. Vieitez, T.I. Ivanov, C.A. de Lange, W. Ubachs, A.N. Heays, B.R. Lewis, G. Stark,
Interactions of the 3ppu c1Pu(v=2) Rydberg-complex member in isotopic N2
J. Chem. Phys. 128 134313 (2008)
M.O. Vieitez, T.I. Ivanov, W. Ubachs, B.R. Lewis, C.A. de Lange,
On the complexity of the absorption spectrum of molecular nitrogen
J. Molecular Liquids 141, 110-117 (2008)
M.O. Vieitez, T.I. Ivanov, J.P. Sprengers, C.A. de Lange, W. Ubachs,
B.R. Lewis, G. Stark
Quantum-interference effects in the
o1Pu(v=1) - b1Pu(v=9)
Rydberg-valence complex of molecular nitrogen
Mol. Phys. 105 (2007) 1543-1557
B.R. Lewis, S.T. Gibson, J.P. Sprengers,
W. Ubachs,
A. Johansson, C.-G. Wahlstrom,
Lifetime and predissociation yield of 14N2
b1Pu(v=1) revisited: effects of rotation
J. Chem. Phys. 123 (2005) 236101
J.P. Sprengers,
E. Reinhold,
W. Ubachs,
K.G.H. Baldwin,
B.R. Lewis,
Optical observation of the 3ssg F3Pu
Rydberg state of N2
J. Chem. Phys. 123 (2005) 144315.
J.P. Sprengers,
W. Ubachs,
K.G.H. Baldwin
Isotopic variation of experimental lifetimes for the lowest 1Pu states in N2
J. Chem. Phys. 122 (2005) 144301
J.P. Sprengers, A. Johansson, A. L'Huillier, C.-G. Wahlstrom,
B.R. Lewis, W. Ubachs
Pump-probe lifetime measurements on singlet ungerade states in molecular nitrogen
Chem. Phys. Lett. 389 (2004) 348-351
J.P. Sprengers, W. Ubachs, A. Johansson, A. L'Huillier, C.-G. Wahlstrom,
R. Lang, B.R. Lewis, S.T. Gibson
Lifetime and
predissociation yield of 14N2 b1Piu(v=1)
J. Chem. Phys. 120 (2004) 8973-8978
J.P. Sprengers, W. Ubachs, K.G.H. Baldwin, B.R. Lewis, W.-U.L. Tchang-Brillet
Extreme ultraviolet laser excitation of isotopic molecular nitrogen:
the dipole allowed spectrum of 15N2 and
14N15N
J. Chem. Phys. 119 (2003) 3160-3173
A. de Lange, R. Lang, W.J. van der Zande, W. Ubachs
Highly excited states of gerade symmetry in molecular nitrogen
J. Chem. Phys. 116 (2002) 7893-7901
W. Ubachs, R. Lang, I. Velchev, W.-U L. Tchang-Brillet, A. Johansson,
Z.S. Li, V. Lokhnygin, C.-G. Wahlstrom
Lifetime measurements on the
c4' 1Su+, v=0,1 and 2
states of molecular nitrogen
Chem. Phys. 270 (2001) 215-225
W. Ubachs, I. Velchev, A. de Lange
Predissociation in b1Pu, v (v=1,4,5,6) levels of N2
J. Chem. Phys. 112 (2000) 5611-5616
A. de Lange, W. Ubachs
Observation of the y1Pg-c4'
1Su+
and k1Pg-c4'
1Su+ systems of N2
Chem. Phys. Lett. 310 (1999) 471
W. Ubachs
Predissociative decay of the
c'41Su+,
v=0 state of molecular nitrogen
Chem. Phys. Lett. 268 (1997) 201
W. Ubachs, K.S.E. Eikema, W. Hogervorst
Narrowband extreme ultraviolet radiation tunable in the range 90.5-95 nm:
application to spectroscopy of N2
Appl. Phys. B57 (1993) 411-416
P.F. Levelt, W. Ubachs
XUV-laser spectroscopy on the c4' 1Su+,
v=0 and c3 1Pu, v=0 Rydberg states of N2
Chem. Phys. 163 (1992) 263
W. Ubachs, L.M. Tashiro, and R.N. Zare
Study of the N2 b1Pu state
via 1+1 multiphoton ionization
Chem. Phys. 130 (1989) 1
Papers on the photo-dissociation spectrum of molecular oxygen
S. Hannemann, G.R. Wu, E.-J. van Duijn, W. Ubachs, Ph. Cosby,
Pressure broadening and fine-structure dependent predissociation
in oxygen B3Su-, v=0
J. Chem. Phys. 123, 174318 (2005)
S. Hannemann, E.-J. van Duijn, W. Ubachs,
Deep-UV high resolution cavity ring-down
spectroscopy of the Schumann-Runge bands in
16O2 and 18O2 at wavelengths 197 - 203 nm
J. Mol. Spectr. 232 (2005) 33-38
Papers on the photo-dissociation spectrum of carbon dioxide
D. Ityaksov, H. Linnartz, W. Ubachs,
Deep-UV absorption and Rayleigh scattering of carbon dioxide
Chem. Phys. Lett. 462, 31-34 (2008)
5. Molecular photoabsorption of atmospheric molecules and satellite data retrieval
We are in the process of investigating absorption by the molecular constituents of the Earth's atmosphere, one the one hand the major species, on the other hand trace gases. Laser-based cavity ring-down absorption is applied to determine the spectra of the oxygen atmospheric bands, even including the spectra of the isotopes. An example of a very weak absorption band of O2 is given in the figure (see also
Paper).

The absorption spectrum of molecular oxygen in the yellow range
Papers on the absorption spectrum of molecular oxygen
H. Naus, A. de Lange, W. Ubachs
b1Sg+ -
X3Sg- (0,0)
band of oxygen isotopomers in relation to tests
of the symmetrization postulate in 16O2
Phys. Rev. A56 (1997) 4755
H. Naus, S.J. van der Wiel, W. Ubachs
Cavity-ring-down spectroscopy on the
b1Sg+ -
X3Sg- (1,0)
band of oxygen isotopomers
J. Mol. Spectr. 192 (1998) 162-168
H. Naus, K. Navaian, W. Ubachs
The gamma-band of 16O2, 16O17O,
17O2 and
18O2
Spectrochimica Acta 55, (1999) 1255
H. Naus, W. Ubachs
The b1Sg+ - X3Sg- (3,0) band of
16O2 and 18O2
J. Mol. Spectr. 193 (1999) 442-445
Water vapour is an extremely important minor constituent in the Earth atmosphere. It contributes for a large fraction to the uptake of infrared radiation making up for a large fraction of the absorption part of the Earth radiation budget. Absorptions in the visible (vibrational overtone bands) are much weaker, but nevertheless contribute. In addition the absorption bands in the visible are of importance, because those can be used for retreival of satellite information. As an example we show the water vapour spectrum in the yellow range.

The water vapour absorption spectrum in the yellow wavelength range
These laboratory data on the water vapor absorption spectrum were used in novel retrieval algorithms to determine the column density of water vapor in the Earth atmosphere.

Comparison of GOME-retrieved precipitable water columns with ECMWF expected values
For this study data were used from the
GOME satellite by ESA with a spectrometer aboard looking into nadir.
Papers on the absorption spectrum of water vapor and the O18 and O17 isotopes:
H. Naus, W. Ubachs, P.F. Levelt, O.L. Polyansky, N.F. Zobov, J. Tennyson
Cavity-Ring-Down spectroscopy on water vapour in the range 555-604 nm
J. Mol. Spectr. 205 (2001) 117-121
M. Tanaka, M. Sneep, W. Ubachs, J. Tennyson,
Cavity-Ring-Down Spectroscopy of H218O in the 16570 -- 17120 cm-1 frequency range
J. Mol. Spectr. 226 (2004) 1-6
O. Naumenko, M. Sneep, M. Tanaka, S.V. Shirin, W. Ubachs, J. Tennyson,
Cavity ring-down spectroscopy of H217O in the range 16570 -17125 cm-1
Supplementary Material
J. Mol. Spectr. 237, 63-69 (2006)
Papers on the water vapor data retrieval from GOME satellite data:
A.N. Maurellis, R. Lang, W. van der Zande, I. Aben, W. Ubachs
Precipitable water column retrieval from GOME data
Geoph. Res. Lett. 27 (2000) 903-906
R. Lang, A.N. Maurellis, W.J. van der Zande, I. Aben, J. Landgraf, W. Ubachs
Forward modeling and retrieval of water vapor from GOME:
Treatment of narrow band absorption spectra
J. Geoph. Res. 107 D (2002) doi:10.1029/2001JD001453
S. Hannemann, G.R. Wu, E.-J. van Duijn, W. Ubachs, Ph. Cosby,
Pressure broadening and fine-structure dependent predissociation
in oxygen B3Su-, v=0
J. Chem. Phys. 123, 174318 (2005)
S. Hannemann, E.-J. van Duijn, W. Ubachs,
Deep-UV high resolution cavity ring-down
spectroscopy of the Schumann-Runge bands in
16O2 and 18O2 at wavelengths 197 - 203 nm
J. Mol. Spectr. 232 (2005) 33-38
D. Ityaksov, H. Linnartz, W. Ubachs,
Deep-UV absorption and Rayleigh scattering of carbon dioxide
Chem. Phys. Lett. 462, 31-34 (2008)

The absorption spectrum of molecular oxygen in the yellow range
H. Naus, A. de Lange, W. Ubachs
b1Sg+ -
X3Sg- (0,0)
band of oxygen isotopomers in relation to tests
of the symmetrization postulate in 16O2
Phys. Rev. A56 (1997) 4755
H. Naus, S.J. van der Wiel, W. Ubachs
Cavity-ring-down spectroscopy on the
b1Sg+ -
X3Sg- (1,0)
band of oxygen isotopomers
J. Mol. Spectr. 192 (1998) 162-168
H. Naus, K. Navaian, W. Ubachs
The gamma-band of 16O2, 16O17O,
17O2 and
18O2
Spectrochimica Acta 55, (1999) 1255
H. Naus, W. Ubachs
The b1Sg+ - X3Sg- (3,0) band of
16O2 and 18O2
J. Mol. Spectr. 193 (1999) 442-445

The water vapour absorption spectrum in the yellow wavelength range

Comparison of GOME-retrieved precipitable water columns with ECMWF expected values
H. Naus, W. Ubachs, P.F. Levelt, O.L. Polyansky, N.F. Zobov, J. Tennyson
Cavity-Ring-Down spectroscopy on water vapour in the range 555-604 nm
J. Mol. Spectr. 205 (2001) 117-121
M. Tanaka, M. Sneep, W. Ubachs, J. Tennyson,
Cavity-Ring-Down Spectroscopy of H218O in the 16570 -- 17120 cm-1 frequency range
J. Mol. Spectr. 226 (2004) 1-6
O. Naumenko, M. Sneep, M. Tanaka, S.V. Shirin, W. Ubachs, J. Tennyson,
Cavity ring-down spectroscopy of H217O in the range 16570 -17125 cm-1
Supplementary Material
J. Mol. Spectr. 237, 63-69 (2006)
A.N. Maurellis, R. Lang, W. van der Zande, I. Aben, W. Ubachs
Precipitable water column retrieval from GOME data
Geoph. Res. Lett. 27 (2000) 903-906
R. Lang, A.N. Maurellis, W.J. van der Zande, I. Aben, J. Landgraf, W. Ubachs
Forward modeling and retrieval of water vapor from GOME:
Treatment of narrow band absorption spectra
J. Geoph. Res. 107 D (2002) doi:10.1029/2001JD001453