| The Roos Lab | Home | People | Research | Publications | Covers | Open positions | Contact |
Viral mechanics

Click for more AFM schematics |
|||
| AFM nanoindentation animation. This animation shows how to probe the mechanical properties of a single virus particle. Click play on the image to display the movie in this window or click here to download the animation to your computer. |
|
To elucidate the stabilization process which occurs during the maturation of Herpes Simplex Virus Type 1 (HSV1) nuclear capsids, we used biochemical and nanoindentation approaches to analyze the structural and mechanical properties of these capsids. From our AFM measurements we conclude that HSV1 capsids are stabilized after removal of the scaffolding proteins, and that this stabilization is triggered by the packaging of DNA, however it is independent of the actual presence of DNA. Read more in PNAS. |
 |
Herpes Simplex capsid imaged by
atomic force microscopy, revealing the
capsomers on top of the icosahedral capsid. Hexons can clearly be
distinguished as well as the three holes left by the pentons, which
were removed by Guanidine . |
|
AFM images of a T=3 and T=4 Hepatitis B viral capsid. The lateral height profiles show the differences in diameter [PNAS, 2008, 105, 9216]. |
 |
In addition to the importance of Hepatitis B virus (HBV) in human health, there is growing interest in adapting HBV and other viruses for drug delivery and other nanotechnological applications. In both of these contexts a precise biophysical characterization of these large macromolecular particles is fundamental. HBV capsids are particularly interesting as they exhibit two distinct icosahedral geometries, T=3 and T=4, nominally composed of 90 and 120 dimers. The figure shows atomic force microscopy (AFM) images of both morphologies. Nanoindentation experiments by AFM indicate that both capsids have similar stabilities and the measurements yielded a Young’s modulus of approx. 0.3 GPa. Furthermore, it was shown that no material fatigue can be observed upon multiple indentations at small forces. Read more on the AFM and the related mass spectrometry measurements [PNAS 2008, 105, 9216]. |
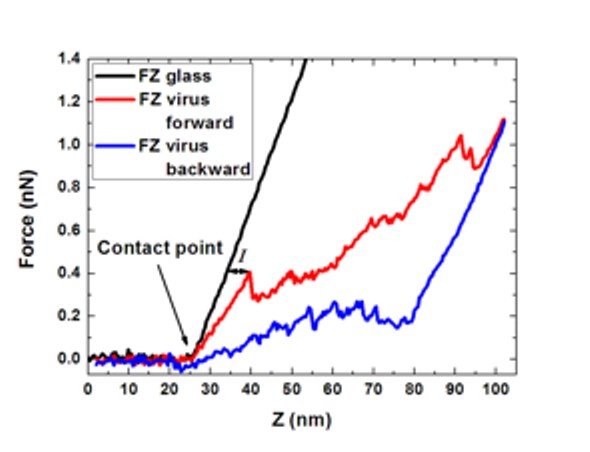
Nanoindentation
measurements start with
recording a high resolution image of the virus. Subsequently the AFM
tip is
directed to the particle centre and the virus is indented. The recorded
FZ
curves (see image) allow for determining (i) the spring constant, from
the
initial linear part of the curve, (ii) the breaking force, the point
where a
drop in the force occurs and (iii) the indentation at which this
occurs. The
field of force spectroscopy measurements on viruses by AFM based
nanoindentation experiments is relatively new. For an overview of
the current literature please click here [Nature Physics, Vol.
6, p. 733, 2010]. Force-distance
(FZ) curves of indentation
of a viral capsid. Next to the reference curve FZ glass, the forward
(loading)
and backward (unloading) curves are shown. The hysteresis between these
curves
shows the irreversibility of indentation. Indentation is denoted by I [Adv. Mat. 2009, 21, 1187].
|
|
|

