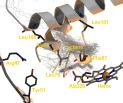
Product formation for hydroxylation of octane and lauric acid by CYP102A1 wild-type and active site mutants F87A, L188Q/A74G and F87V/L188Q/A74G, were rationalized using a combination of binding statistics from molecular dynamics simulation and barrier energies from quantum mechanical calculations. BM3 typically hydroxylates on sub-terminal (-1, -2, -3) but not the terminal () positions. The known carboxylic anchoring site Y51/R47 and hydrophobic interactions and steric exclusion, mainly by F87, play a role in determining the binding modes of the substrates. Electrostatic interactions between the protein and the substrate strongly modulate the substrate’s regio-dependent reactivity. The combination of the binding statistics and the reactivity of the substrates determines the product formation. Trends observed in experimentally determined product formation for octane and lauric acid by the WT enzyme and the three active site mutants are qualitatively explained. The combination of binding statistics and barrier energies is a valuable tool to rationalize substrate binding and product formation and constitutes an important step towards quantitative prediction of product ratios.