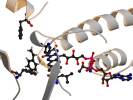
Styrene monooxygenase StyAB has been characterized earlier as a two-component FAD- and NADH-dependent monooxygenase. Since a crystal structure is lacking so far, a structural model based on p-hydroxybenzoate hydroxylase pHBH and phenol hydroxylase has been calculated to gain further insight the catalytic site architecture of StyA. Mutations in supposed important site were built that affirmed functional similarities of StyA and pHBH. The substrate-binding pocket has been shown to be exclusively hydrophobic, and two phenylalanine side chains stabilize the aromatic substrate styrene. Introducing hydrophilic residues or omitting the aromatic side chain leads to significant decrease in activity. Two loops flanking substrate and cofactor were identified having distinct functions in rigidity of the protein and allowing proper distances for catalytic performance. The entire catalytic mechanism has not been fully understood yet, but using a basic structural alignment allowed a deeper [insight] in catalytically important amino acid in StyA.